NuVasive MAGEC System Lawsuit
A NuVasive MAGEC system lawsuit may be an option for people with early onset scoliosis (EOS) who experienced serious complications related to the magnetic rods. The NuVasive MAGEC System is used to treat patients with early onset scoliosis (EOS) without the need for regular distraction (lengthening) surgery. However, the magnetic growing rods are reportedly linked to serious complications, including device fracture and metallosis. Affected patients and their families may be eligible to pursue compensation for damages with the help of a defective medical device attorney.
If you or a loved one have been adversely affected by a potentially defective medical device, contact Attorney Group to learn about your options. We offer free, no obligation consultations. We can help answer your questions, and if you choose to pursue a claim we can connect you with an affiliated attorney who can assist you throughout the legal process.

Have You Seen a NuVasive MAGEC System Lawsuit Commercial?
You may have seen a NuVasive MAGEC system lawsuit commercial on television and wondered whether you or a loved one have been affected by the potentially defective medical devices and, if so, whether you are eligible to pursue a claim against the manufacturer or distributor. The purpose of this article is to provide you with additional information about recalls, lawsuits, and settlements involving potentially defective magnetic growing rods used to treat early onset scoliosis so that you have a better understanding of your options.
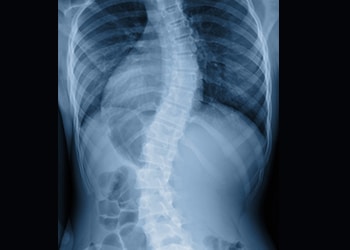
What is Early Onset Scoliosis?
Early Onset Scoliosis (EOS) is a form of scoliosis — curvature of the spine more than 10 degrees — identified in patients 10 years-old or younger.
According to the Scoliosis Research Society, causes of EOS may include:
- Idiopathic – Curves for which there is no apparent cause
- Congenital – Development of vertebrae in the womb that is sometimes associated with cardiac and renal abnormalities
- Neuromuscular – Disorders including spinal muscular atrophy, cerebral palsy, spina bifida, and brain or spinal cord injury
- Syndromic – Syndromes associated with EOS may include Marfans, Ehlers-Danlos, neurofibromatosis, Prader-Willi, as well as other connective tissue disorders and bone dysplasia
In some cases, a patient may have genetic or inherited EOS in which one or both parents had a gene that is present in their child.
Although patients with mild to moderate scoliosis do not typically have more back pain than people who don’t have the condition, scoliosis that is severe or caused by an abnormality of the spinal cord could lead to back pain.
Complications of EOS, and other forms of scoliosis, may include difficulty breathing and problems with weight gain and growth development. Some deformities of the spine may pinch the spinal cord and lead to neurological symptoms including pain, numbness or weakness.
Early Onset Scoliosis Treatment
A wide range of treatments are available for patients with EOS; however, a patient’s treatment needs are often unique to each child and their doctor.
Treatment options may include observation, bracing or casting, or surgery.
Observation
A patient’s doctor may initially choose to monitor the behavior of the curve with clinic visits and x-ray exams. If the curve of the spinal cord worsens or grows, other treatment options may be appropriate.
Bracing or Casting
In some cases, bracing or casting may help with spinal growth while minimizing any increase in the scoliosis. Braces rarely permanently correct the issue; however, a brace can potentially slow the progression of the curve and allow the child to grow before a surgical procedure is done.
Surgery
If the brace or cast treatment fails, or if the curve cannot be corrected with a cast or brace, a doctor may recommend a surgical procedure to correct the curve. Traditional or magnetic growing rods may be used on either side of the spine with spine anchors to correct some of the scoliosis and to help the spine grow during treatment.
Traditional rods require small, invasive surgical procedures approximately every six months, while magnetic rods require no additional surgeries, and a small magnet is used to lengthen the rods while the child is awake.
Complications associated with surgery may include slow or poor healing of the wound, movement or breakage of anchors, spinal injury, deep infection, and surgical failure.
What is the NuVasive MAGEC System?
The NuVasive MAGEC System is a type of magnetic growing rod system used to treat EOS.
Headquartered in San Diego, NuVasive is a medical device company focused on developing minimally disruptive surgical products and procedures for the spine.
In September 2017, the U.S. Food and Drug Administration determined (in accordance to provisions of the Federal Food, Drug, and Cosmetic Act) that an updated version of the NuVasive MAGEC System was substantially equivalent to other legally marketed devices and did not require approval of a premarket approval application.
NuVasive MAGEC System Complications
The NuVasive MAGEC System may be an appropriate treatment option for some patients who suffer from EOS. However, some reports indicate serious complications associated with the magnetic growing rods.
A number of peer-reviewed articles have examined potential complications related to the MAGEC system to treat EOS. Those complications may include:
- Metallosis – According to articles published in The Bone and Joint Journal in December 2016 and June 2017, researchers noted significant tissue metallosis surrounding some implants associated with metal debris from the growing rods. Metallosis occurs when metal debris enters the soft tissues of the body. Symptoms of metallosis may include pain, pseudo tumors, and tissue death.
- Actuator pin fracture – Subtle fractures may occur in the distraction element of the growing rods. According to an analysis of two case reports published by The Spine Journal in 2016 and an analysis of explanted devices published in the UK journal Spine, researchers noted evidence of a number of cases in which drive pins within the devices’ actuator fractured.
- Revision surgery – In some cases, revision surgery may be necessary to correct any issues with the growing rods. Revision surgeries often carry an increased risk in a patient’s vulnerability to other complications in addition to any added financial burdens.
Additionally, surgery to implant the MAGEC system is considered to be a major operation. Genearl risks associated with the devices may include bending, loosening, moving, or breaking of implant; allergic reaction to implant materials (such as titanium); worn skin; infection or surgical wound complications; and pain or discomfort.
NuVasive MAGEC System and Metallosis
Metallosis is most commonly associated with metal-on-metal hip implants; however, metal particles from any type of metal implant (such as a growing rod) could potentially cause a reaction around the area of the device.
According to the FDA, such a reaction could lead to the deterioration of the soft tissues surrounding the device and possible failure of the device. Likewise, metal ions from the growing rods could enter the bloodstream and potentially lead to other complications.
Additional complications associated with metallosis may include:
- General hypersensitivity reaction (skin rash)
- Cardiomyopathy
- Neurological changes, including auditory or visual changes
- Psychological status change (such as depression or cognitive impairment)
- Renal function impairment
- Thyroid dysfunction, including neck discomfort, fatigue, weight gain or feeling cold
Lauren A. on May 16, 2016
Attorney Group reviewed by:"These guys are a pleasure to work with -- very strategic and very responsive, which makes for a great business partner! I can tell that they are passionate about making sure all clients get the attention and expertise they deserve."Rating: 5 ★★★★★
Has There Been a NuVasive MAGEC System Recall?
Although there has not been a NuVasive MAGEC System recall, complications (such as pain, infection, metallosis and need for revision surgery) may lead to serious injury.
Affected individuals who suffered serious complications as a result of potentially defective growing rods could pursue a claim against any manufacturers that failed to disclose known risks associated with the devices.
Is There a NuVasive MAGEC System Class Action Lawsuit?
At the time of this article’s publication, a NuVasive MAGEC System class action lawsuit action has not been filed. NuVasive MAGEC System lawsuit attorneys are doubtful that a class action will be certified for patients who are adversely affected by the device. Instead, if multiple lawsuits are filed against the manufacturers, alleging injuries and other damages caused by adjustable valves, shunts and similar medical devices, it is anticipated that these potential lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court it is called multidistrict litigation (MDL), and on a state level it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical devices.
In most cases that proceed in an MDL or state court consolidated proceedings, after a certain period of time initial trials, also known as bellwether trials, take place. The purpose of these trials is for the parties to get an idea of the types of evidence and arguments that will made, as well as to see how juries will respond to the evidence and arguments. After a certain number of cases have been tried, the parties are in a better position to determine whether a case can be settled.
Have There Been NuVasive MAGEC System Lawsuit Settlements?
Some defective medical device lawsuits may settle early in the claims process. However, it is not expected that there will be any NuVasive MAGEC System lawsuit settlements at this time.
Instead, it is expected that adjustable valve and shunt lawsuits will be consolidated in federal court through an MDL. NuVasive MAGEC System lawsuit attorneys note that the outcome of any case is never guaranteed, and past results are not necessarily predictive of future outcomes.
NuVasive MAGEC System Lawsuit News
- 2017Article published in The Bone and Joint Journal examines reports of implant failures and metallosis associated with the NuVasive MAGEC System.
- 2016Research published in Spine (an international, peer-reviewed, bi-weekly periodical focused on the spine and spinal disorders), indicates a "combination of high volumes of titanium wear debris alongside O-ring seal damage," potentially accounting for the occurrence of metallosis found around some MAGEC rods.
How a NuVasive MAGEC System Lawsuit Attorney Can Help
Medical device makers have a duty to provide safe products. If there are risks of harm associated with their products, they also must provide adequate warnings. If a medical device maker fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by a defective NuVasive MAGEC System may be eligible to recover money for:
 Medical Expenses
Medical Expenses
 Lost Wages
Lost Wages
 Pain and Suffering
Pain and Suffering
The families of those who have died may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.





