St. Jude Defibrillator Battery Lawsuit
A St. Jude defibrillator battery lawsuit may be an option for patients who have been injured due to premature battery loss of the heart devices. In addition to a safety communication issued by the U.S. Food and Drug Administration (FDA), two patients have reportedly died as a result of premature battery loss associated with St. Jude Medical Implantable Cardioverter Defibrillators (ICDs) and Cardiac Resynchronization Therapy Defibrillators (CRT-Ds). Affected patients and their families may be eligible to pursue compensation with the help of a defective medical device lawyer.
For more information, contact Attorney Group today. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case we can connect you with an affiliated St. Jude defibrillator battery lawsuit attorney who can assist you throughout the legal process.

Have You Seen a St. Jude Defibrillator Battery Lawsuit Commercial?
You may have seen a St. Jude defibrillator battery lawsuit commercial on television and wondered whether you or a loved one have been affected by premature battery loss associated with ICDs and CRT-Ds and, if so, whether you are eligible to pursue a claim against the manufacturer or others. The purpose of this article is to provide you with additional information so that you have a better understanding of your options.
What is a St. Jude Defibrillator?
Implantable Cardioverter Defibrillator
Implantable cardioverter defibrillators (ICDs) are electronic, battery-powered devices used to monitor a patient’s heart rate. The small device is placed just below the skin in the chest or abdomen, and thin wires connect the ICD to the heart.
When an abnormal heart rate is detected, the defibrillator delivers an electric shock to restore a normal heartbeat if a patient’s is beating too rapidly. Newer-model ICDs sometimes function as a pacemaker as well and can stimulate the heart if the heart rate is too slow.
According to the American Heart Association (AHA), ICDs have been very effective in preventing sudden death in patients with known heart conditions, including ventricular tachycardia or fibrillation.
Cardiac Resynchronization Therapy Defibrillator
Cardiac resynchronization therapy defibrillators (CRT-Ds) are heart devices designed to treat symptoms associated with heart failure by sending specially timed electrical impulses to improve the timing and resynchronize the pumping action of the heart’s left and right ventricles.
Should a dangerously fast heart rhythm occur, CRT-Ds shock the heart rhythm back to normal. CRT-Ds consist of a pulse generator that contains a battery and other electric circuitry in addition to a set of wires (also known as leads) that deliver the electric stimulation to the patient’s heart.
Who Uses an ICD or CRT-D?
Doctors may recommend an ICD if a patient is at risk of a life-threatening ventricular arrhythmia due to a variety of conditions, including:
- A previous ventricular arrhythmia
- A previous heart attack
- Survival of sudden cardiac arrest
- Long QT syndrome, a heart rhythm disorder that can potentially cause rapid, chaotic heartbeats
- Brugada syndrome, a genetic disease characterized by abnormal electrocardiogram (EKG) findings and an increased risk of sudden cardiac death
- Other congenital heart disease or underlying conditions for sudden cardiac arrest
According to St. Jude Medical, patients may need a CRT-D if they meet the following qualifications:
- Previously have had or are at risk for having ventricular tachycardia or ventricular fibrillation, causing the heart to beat too fast
- Damage to the pumping action of the heart caused by a heart attack
- Heart failure
CRT-Ds are essentially ICDs that provide added therapy to treat patients with the potential for heart failure.
Lauren A. on May 16, 2016
Attorney Group reviewed by:"These guys are a pleasure to work with -- very strategic and very responsive, which makes for a great business partner! I can tell that they are passionate about making sure all clients get the attention and expertise they deserve."Rating: 5 ★★★★★
ICD and CRT-D Risks
Risks associated with ICD implantation may include:
- Infection at the implant site
- Allergic reaction to medications used during the procedure
- Swelling, bleeding or bruising where the device was implanted
- Damage to the vein where the ICD leads are placed
- Bleeding around the heart, which can be life-threatening
- Blood leakage through the heart valve where the ICD lead is placed
- Collapsed lung
Similar risks are involved with CRT-Ds, including infection, lead dislodgement and perforation.
ICD and CRT-D Battery Failure
In October 2016, St. Jude Medical issued a press release notifying physicians of the risk of premature battery depletion associated with lithium deposits in a subset of St. Jude Medical ICD and CRT-D devices manufactured before May 23, 2015. St. Jude asserts that the occurrence of battery depletion is rare, however, the company is providing information so that physicians can care for their patients should they have an affected device.
According to an article issued by Reuters on October 11, 2016, St. Jude Medical said it would recall roughly 400,000 of the implanted heart devices due to risk of possible rapid battery failure. In some cases, full battery depletion can occur within a day to a few weeks after the patient receives an ERI alert. Two deaths have been reportedly linked to the loss of defibrillation therapy as a result of premature battery loss.
Has There Been a St. Jude Defibrillator Battery Recall?
St. Jude Medical has issued a recall notice for certain models of the Fortify, Unify, and Assura ICDs and CRT-Ds due to reports of rapid battery failure caused by deposits of lithium in the battery, leading the devices to short circuit. The U.S. Food and Drug Administration (FDA) and St. Jude Medical are alerting patients, patient-caregivers and physicians to respond immediately to Elective Replacement Indicator (ERI) alerts.
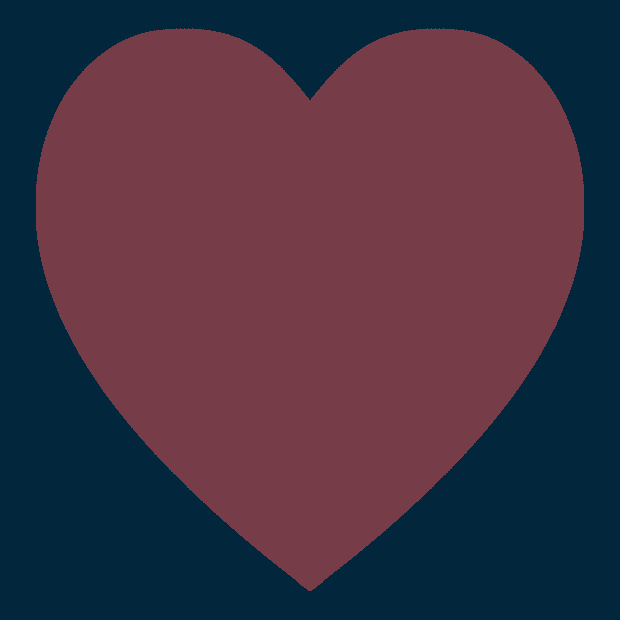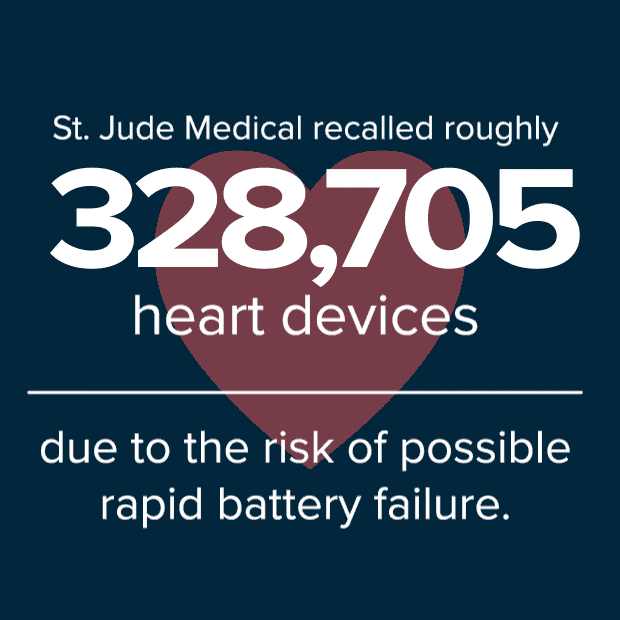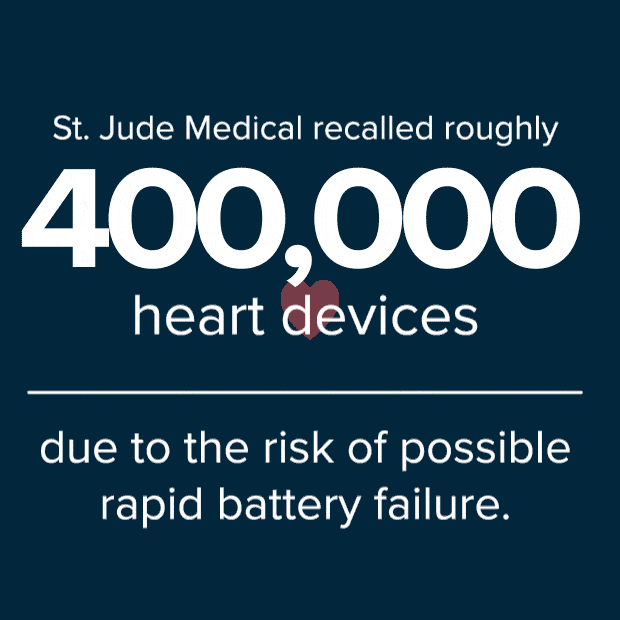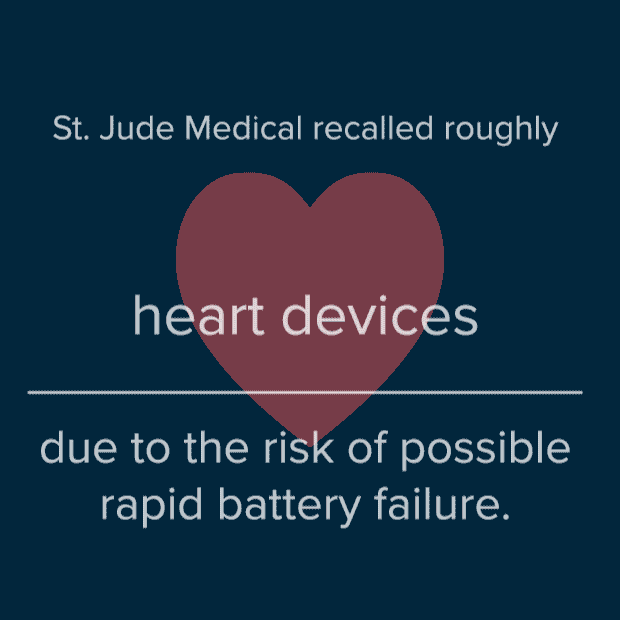
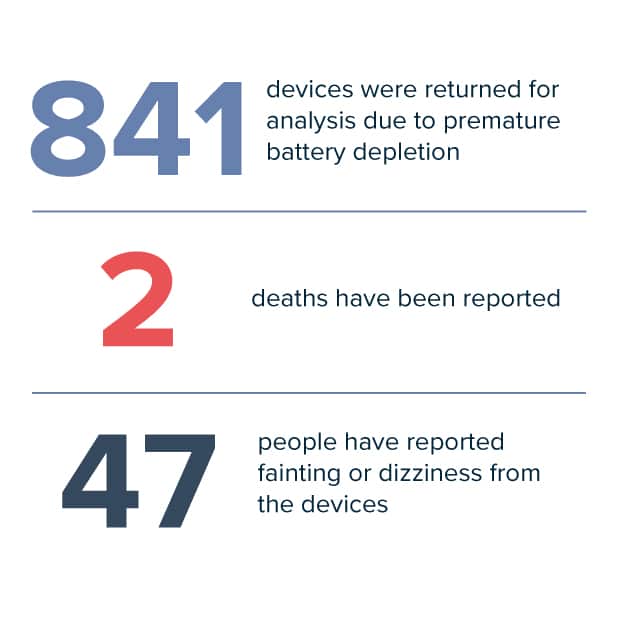
To date, 841 devices were returned for analysis due to premature battery depletion. In addition to the two reported deaths, 10 people have reported fainting and 37 have reported dizziness from devices that could not provide adequate pacing therapy due to battery depletion.
| Recalled devices were manufactured before May 2015 and include the following ICD and CRT-D models: | |
|---|---|
| Fortify VR | Fortify Assura ST DR |
| Fortify ST VR | Unify |
| Fortify Assura VR | Unify Quadra |
| Fortify Assura ST VR | Unify Assura |
| Fortify DR | Quadra Assura |
| Fortify ST DR | Quadra Assura MP |
| Fortify Assura DR | |
The FDA warns that battery depletion may not always be reported to the manufacturer, so the actual number of devices that could potentially experience premature battery depletion is unknown. Nearly 350,000 affected devices remain actively implanted worldwide.
FDA Recommendations
In a safety communication issued by the FDA, patients are advised to contact their physician if they feel a vibratory alert and to register for home monitoring to alert the patient’s physician. If symptoms of lightheadedness, dizziness, loss of consciousness, chest pain, or severe shortness of breath occur, patients are advised to seek medical attention immediately.
Likewise, health care providers are asked not to implant unused affected devices and to talk with their patients who have affected devices about the risk of battery depletion. Health care professionals are to continue to conduct follow-up visits with patients who are implanted with an affected devices and to immediately replace the device, should an ERI alert occur.
Is There a St. Jude Defibrillator Battery Class Action?
There is no St. Jude defibrillator battery class action pending as of October 2016. St. Jude defibrillator battery lawsuit attorneys are doubtful that a class action will be certified for patients who are adversely affected by the heart devices. Instead, if multiple St. Jude defibrillator battery lawsuits are filed against the device manufacturers alleging injuries and other damages caused by St. Jude Medical defibrillators and similar heart devices, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court it is called multidistrict litigation (MDL), and on a state level it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical devices.
Have There Been St. Jude Defibrillator Battery Lawsuit Settlements?
A St. Jude defibrillator battery lawsuit attorney notes that some cases settle early in the claims process, but it is not expected that there will be early St. Jude defibrillator battery lawsuit settlements. In most cases that proceed in an MDL or state court consolidated proceedings, after a certain period of time initial trials, also known as bellwether trials, take place. The purpose of these trials is for the parties to get an idea of the types of evidence and arguments that will made, as well as to see how juries will respond to the evidence and arguments. After a certain number of cases have been tried, the parties are in a better position to determine whether a case can be settled.
It is expected that St. Jude defibrillator settlements will follow this pattern, although the outcome of any case is never guaranteed and past results are not necessarily predictive of future outcomes.
St. Jude Defibrillator Battery Lawsuit News
- April 2017The FDA sends warning letter to St. Jude Medical for failing to adequately investigate problems associated with implantable defibrillator batteries.
- October 2016Two deaths are reported to have been linked to loss of defibrillation therapy as a result of premature battery depletion.
- October 2016The FDA issues a safety communication regarding the recall of St. Jude Medical ICD and CRT-D devices, providing recommendations for health care providers, patients and caregivers.
- October 2016St. Jude Medical issues an Important Medical Device Advisory notifying doctors and health care providers of the risk of premature battery depletion associated with the company’s ICD and CRT-D devices manufactured before May 2015.
How a St. Jude Defibrillator Battery Lawsuit Can Help
Medical device makers have a duty to provide safe products. If there are risks of harm associated with their devices, they also must provide adequate warnings. If a device maker fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by a defective heart defibrillator may be eligible to recover money for:
 Medical Expenses
Medical Expenses
 Lost Wages
Lost Wages
 Pain and Suffering
Pain and Suffering
The families of those killed may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.





