Stryker Hip Recall Lawyers can help you today if you’ve had a Stryker hip replacement recalled. The FDA approved the Stryker Rejuvenate systems between 2008 and 2009. Since they were “substantially similar” to previously approved technologies, extensive tests before marketing were not required. Stryker was only required to perform post-market surveillance.
Voluntary recall
Hip implants usually have a one-piece neck and stem, but the Stryker Rejuvenate system includes six stems and sixteen necks and promised longer-lasting support for younger patients. The Stryker coats its stems with titanium, and the necks are cobalt and chromium; because of this, they have been proven to cause the same complications as other metal-on-metal devices. In April 2012, they warned surgeons and hospitals of the real and potential hazard. In June 2012, Stryker initiated a voluntary recall due to the potential for rubbing and corrosion.
Additional Surgeries Required
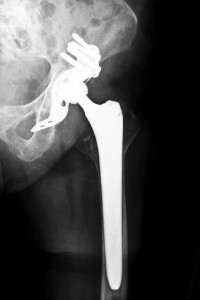
Tens of thousands of people have undergone repeat surgeries to replace failed hip implants. Known as a “revision surgery,” this procedure is complicated and possibly dangerous. The mortality rate among patients climbs 1% to 2.5% from the original surgery.
Despite the risks, patients often have little to no choice if they want to function normally again. Surgeons feel the procedures are hard to perform and leave the patient to a long, hard recovery. The surgery dislocates your hip before the removal of the problematic parts. The cup-shaped cavity is cleaned our and lined with a bone graft. The surgeon’s talent is put to the real test in handling the femur where yet another bone graft may be required.
Potentially Fatal Complications
Most patients are elderly and more prone to complications. Positive outcomes depend on the quality of the damaged and defective bone. Unfortunately, revision surgery is not that promising.
- Deep Vein Thrombosis (DVT) is more likely to occur after hip surgery when clots form in large veins in the leg.
- Myosistis ossification sees bone grow around the joint producing yet more stiffness and pain.
- Other concerns are necrosis, hypothyroidism, osteolysis, infection, and loosening of the implant.
Legal Options & The Need to Hire Stryker Hip Recall Lawyers
Stryker offered to reimburse patients for necessary revision surgery and related expenses. They are working with a third-party claims administrator to manage the reimbursements. People who received the Stryker Rejuvenate should consult their medical professional about possible complications. They may also contact a qualified dangerous-device lawyer to protect their interests. Potential compensation available to you includes:
- Medical expenses including revision surgery and replacement
- Pain and suffering – albeit physical, mental, and/or emotional pain
- Loss of consortium
- Loss of income and promise of income
- Wrongful death damages, in the event of the loss of a loved one
Contact Us For More Information
For more information, see our Stryker Hip Recall Information Page and contact Attorney Group. You can fill out the form on this page, call us at the number listed at the top of the page, or email us at info@attorneygroup.com.
When you contact us, an attorney will follow up with you to speak with you about your case or answer questions that you might have. There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.
See our Frequently Asked Questions page for more information, and contact Attorney Group today.





