
Injured by a Medical Device? Here’s What You Should Know.
Medical devices are specifically designed for medical purposes and are used to diagnose, treat or monitor a patient suffering from a particular disease or disability and improve the […]

Medical devices are specifically designed for medical purposes and are used to diagnose, treat or monitor a patient suffering from a particular disease or disability and improve the […]
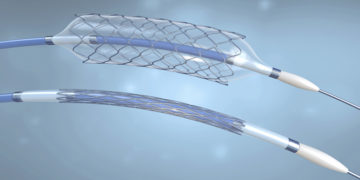
Abbott Vascular is recalling NC Trek RX Coronary Dilatation Catheter and NC Traveler RX Coronary Dilatation Catheters because some balloons from the impacted lots may not deflate as […]
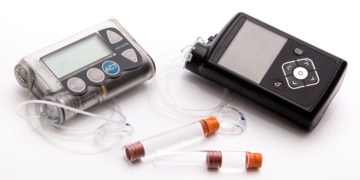
On February 12, the Food and Drug Administration (FDA) announced that Medtronic has issued a recall on certain models of its MiniMed 600 Series Insulin Pumps. The recalled […]
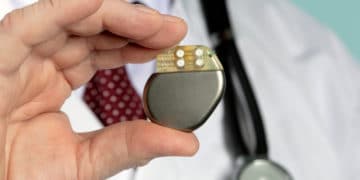
On May 7, 2019, the U.S. Food and Drug Administration (FDA) issued a safety communication about issues with certain Medtronic implantable pacemakers or cardiac resynchronization therapy pacemakers (CRT-Ps). […]

Attorneys are currently investigating allegations of adverse health events associated with a permanent form of birth control known as Essure. After continued monitoring and possible penalties imposed by […]
Researchers reportedly indicate that metal ions released from traditional and magnetically controlled growing rods can potentially “cause localized debris and distribute systemically to settle on distant organs.” Magnetically […]
Abbott Vascular has initiated a voluntary coronary catheter recall for specific lots of three catheters manufactured and distributed between 2015 and 2017. According to the U.S. Food and […]
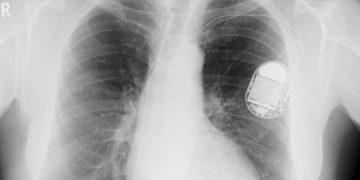
The U.S. Food and Drug Administration (FDA) has reportedly sent a St. Jude defibrillator warning letter accusing the device manufacturer of failing to adequately investigate problems related to […]
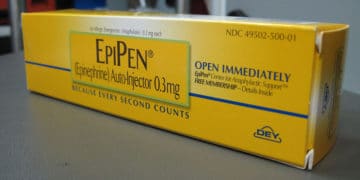
A nationwide Mylan EpiPen recall has been issued by Meridian Medical Technologies for 13 lots of Mylan’s EpiPen and EpiPen Jr Auto-Injectors used for the emergency treatment of severe […]
Philips Healthcare has initiated a recall for the HeartStart MRx Monitor/Defibrillator because of issues with the electrical and battery connections that could prevent the device from powering up, charging […]

A defective breast implant attorney notes that a lawsuit alleges silicone breast implants sold by a Johnson & Johnson subsidiary may leak and lead to serious problems for […]

A Greatbatch Standard Offset Cup Impactor recall has been initiated due to failed sterilization testing of the reusable surgical devices. Devices that have failed such testing can potentially […]
A Centurion Multi-Med Catheter recall has been initiated because the catheters have a potential for excess material to remain at the tip of the catheter, possibly leading to […]

A DePuy Pinnacle hip implant attorney notes that a jury awarded over $1 billion to six plaintiffs in a third case against multinational medical device manufacturer Johnson & […]

A Ventralex Hernia Patch lawyer notes that a lawsuit alleges the devices may be made of materials biologically incompatible with human tissue, potentially leading to severe health complications. […]